Photosolvolysis of bulky (4-hydroxyphenyl)naphthalene derivatives†
Abstract
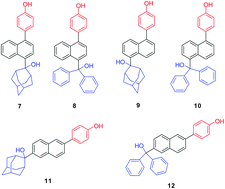
Six new naphthylphenols 7–12, bearing bulky hydroxymethyl substituents on the naphthalene, were synthesized and their photoreactivity was investigated by preparative irradiation, fluorescence measurements, and laser flash photolysis. All derivatives (in S1) undergo deprotonation of the phenolic OH in the aqueous solution. Also, fluorescence quenching with HClO4 in the pH range 2–4 indicates that 7–12 can be protonated in S1. Formation of QMs most probably takes place sequentially, triggered by the phenol deprotonation. However, with the present data, a mechanism that involves simultaneous deprotonation and the loss of OH− cannot be ruled out. Photodehydration takes place only for 7, 9, 11 and 12, delivering the corresponding QMs which react with nucleophilic solvents giving the corresponding photosolvolysis products. The other less likely option for the formation of the observed solvolysis products from 7, 9, 11 and 12 may involve some radical species. Photodehydration of 8 and 10 was not observed which may be due to the anticipated high energy of the corresponding sterically-congested QM8 and QM10. The most efficient photosolvolyses were observed for the 2,6-substituted naphthalenes.

 Please wait while we load your content...
Please wait while we load your content...