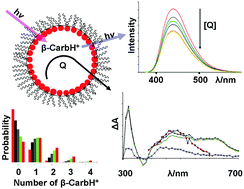
The fluorescence quenching of protonated β-carbolines has been investigated in acidic aqueous solutions and in w/o microemulsions using I−, Br−, Cu2+, SCN−, and Pb2+ as quenchers. It was found that fluorescence quenching by these compounds is much more efficient in water than in microemulsions since quenching in microemulsions depends on the simultaneous occupancy of the water droplets by both fluorophore and quencher. Linear Stern–Volmer plots were obtained in all cases, leading to quenching rate constants of ca. 108–1010 M−1 s−1 in water and ca. 107–108 M−1 s−1 in microemulsions. In the case of quenching by SCN−, ns flash photolysis studies indicate formation of (SCN)2˙− showing that at least part of the quenching process involves an electron transfer mechanism. This indicates that the singlet excited states of the protonated β-carbolines can act as relatively strong oxidants (E° > 1.6 V), capable of oxidizing many species, including the biologically relevant DNA base guanine. The observation of the (SCN)2˙− transient in microemulsions demonstrates that it is possible to have the protonated β-carboline and at least two thiocyanate ions in the same water pool.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...