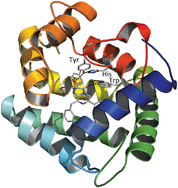
Ca2+-regulated photoproteins are responsible for the bioluminescence of a variety of marine organisms, mostly coelenterates. The photoproteins consist of a single polypeptide chain to which an imidazopyrazinone derivative (2-hydroperoxycoelenterazine) is tightly bound. According to photoprotein spatial structures the side chains of His175, Trp179, and Tyr190 in obelin and His169, Trp173, Tyr184 in aequorin are at distances that allow hydrogen bonding with the peroxide and carbonyl groups of the 2-hydroperoxycoelenterazine ligand. We replaced these amino acids in both photoproteins by residues with different hydrogen bond donor–acceptor capacity. All mutants exhibited luciferase-like bioluminescence activity, hardly present in the wild-type photoproteins, and showed low or no photoprotein activity, except for aeqH169Q (24% of wild-type activity), obeW179Y (23%), obeW179F (67%), obeY190F (14%), and aeqY184F (22%). The results clearly support the supposition made from photoprotein spatial structures that the hydrogen bond network formed by His–Trp–Tyr triad participates in stabilizing the 2-hydroperoxy adduct of coelenterazine. These residues are also essential for the positioning of the 2-hydroperoxycoelenterazine intermediate, light emitting reaction, and for the formation of active photoprotein. In addition, we demonstrate that although the positions of His–Trp–Tyr residues in aequorin and obelin spatial structures are almost identical the substitution effects might be noticeably different.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...