The synthesis of chiral β-aryl-α,β-unsaturated amino alcohols via a Pd-catalyzed asymmetric allylic amination†
Abstract
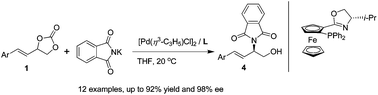
Chiral β-aryl-α,β-unsaturated amino alcohols were synthesized via a Pd-catalyzed asymmetric allylic amination of 4-aryl-1,3-dioxolan-2-one using planar chiral 1,2-disubstituted ferrocene-based phosphinooxazolines as ligands. Under the optimized reaction conditions, a series of substrates were examined and the products were obtained in good to excellent yields (up to 92%) and enantioselectivities (up to 98% ee) under mild reaction conditions. The desired products were determined to be of (R)-configuration and could subsequently be transformed into compounds with interesting biological activity using simple transformations.

 Please wait while we load your content...
Please wait while we load your content...