Jik Chin, Soon Ho Kwon, Kimia Moozeh, Soon Mog So, Alan J. Lough and B. Moon Kim
Org. Biomol. Chem., 2013,11, 8022-8025
DOI:
10.1039/C3OB41582A,
Communication
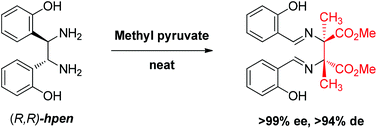
Reaction between 1,2-bis(2-hydroxyphenyl)-ethylenediamine (hpen) and methyl pyruvate gives the diaza-Cope rearrangement product with good yield and excellent stereospecificity. The product containing two chiral quaternary carbon centers is characterized by high performance liquid chromatography and X-ray crystallography. DFT computation provides insight into why the diaza-Cope rearrangement takes place readily with methyl pyruvate but not with other ketones like acetone and substituted acetophenones.