Alkaloid inspired spirocyclic oxindoles from 1,3-dipolar cycloaddition of pyridinium ylides†
Abstract
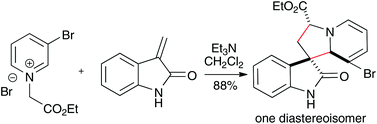
Cycloaddition reactions between pyridinium ylides and 3-alkenyl oxindoles that proceed in high yield and with very good regio- and diastereoselectivity are reported. The resulting cycloadducts have the same stereochemistry of biologically active

 Please wait while we load your content...
Please wait while we load your content...