Dual catalysis by Cu(i): facile single step click and intramolecular C–O bond formation leading to triazole tethered dihydrobenzodioxines/benzoxazines/benzoxathiines/benzodioxepines†
Abstract
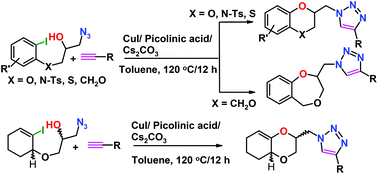
Dual copper catalysis, involving two different reactions, click (alkyne–azide) and carbon–oxygen bond formation (aryl iodide–secondary alcohol) in a single step, is reported. Synthesis of novel benzodioxines (benzodioxanes), benzoxazines, benzoxathiines and benzodioxepines, which feature benzo-condensed six or seven membered rings containing two hetero-atoms attached to a 1,2,3-triazole, is described. As an extension, such compounds were also synthesised by ring opening of epoxide and cyclisation using Cu(I). All the key products have been characterized by single crystal X-ray crystallography.

 Please wait while we load your content...
Please wait while we load your content...