First total synthesis of (+)-apotrisporin E and (+)-apotrientriols A–B: a cyclization approach to apocarotenoids†
Abstract
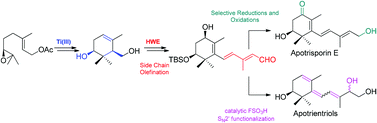
The first total synthesis of the natural apocarotenoids (+)-apotrisporin E (1) and (+)-apotrientriols A and B (2–3) has been accomplished. The structure, relative stereochemistry and the assignation of the absolute configuration have been confirmed. This is a fast and easy access to this family of natural products whose key steps are a diastereoselective cyclization and a HWE olefination to attach the dienic side chain. This work also opens the door to the synthesis of other apocarotenoids such as trisporols and trisporic acids.

 Please wait while we load your content...
Please wait while we load your content...