Helicity discrimination in N,N′-dibenzoyl-1,2,3,4,7,8,9,10-octahydro-1,10-phenanthrolines and their thiono- and selenocarbonyl analogues by inclusion complexation with chiral diols†
Abstract
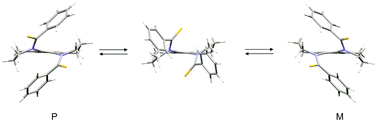
X-ray crystallographic analysis of the title compounds revealed that they assume a folded helical conformation of an approximate C2 symmetry in the solid state. Dithioamide 5b, diselenoamide 5c and monoselenoamide 5d were resolved to enantiomers by inclusion crystallization with optically active diols (TADDOLs). The absolute configuration of the guest molecules in the complexes 5b·6a, 5c·6a and 5d·6a was assigned as P. The optical activity of the resolved compounds is manifested by their CD spectra showing relatively strong Cotton effects in the region of thionoamide and selenoamide n–π* transition. The optically active thiono- and selenoamides are configurationally labile compounds and gradually racemize in solution but they are stable in the form of the inclusion complexes. The first-order kinetics of the racemization in solution allowed us to assign the racemization barriers by the spectropolarimetric measurements.

 Please wait while we load your content...
Please wait while we load your content...