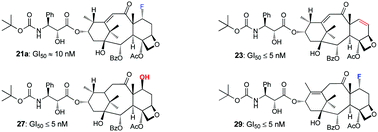
Reaction of 10-deacetylbaccatin III (III) and its 7-TES derivative (IV) with DAST under various conditions resulted in the formation of an array of new fluorinated and non-fluorinated 13-keto taxoid compounds (2a–4a) through a vinylogous pinacol–pinacolone rearrangement. Further fluorination of some of these products (2a, 3a) with NFSi or Selectfluor gave additional derivatives. Sodium borohydride reduction of the 13-keto group of these products (2a, 2b, 3a, 3b, 4a, 8, 9, 11–14) led to a series of 9α-hydroxy taxoid derivatives, which were esterified using the docetaxel side chain employing the corresponding protected β-lactam, followed by deprotection to furnish a library of docetaxel analogs and related compounds. A selected number of synthesized compounds (7, 10, 19a, 19b, 21a, 21b, 23, 27, 29, 34–36) were submitted to the National Cancer Institute (NCI) 60 cell line screening program and tested for cytotoxic properties. Taxoids 19a, 19b, 21a, 21b, 23, 27, 29, 34 and 35 were found to exhibit significant anticancer activity against various cancerous cell lines with 23, 27, and 29 being the most potent compounds, demonstrating GI50 values of ≤5 nM in several assays.

 Please wait while we load your content...
Please wait while we load your content...