Macrocycle synthesis by trimerization of boronic acids around a hexaol template, and recognition of polyols by resulting macrocyclic oligoboronic acids†‡
Abstract
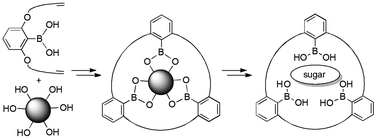
2,6-Bis(alkenyloxy) substituted arylboronic acids can be cyclotrimerized with the help of a hexaol as a template. First, the

 Please wait while we load your content...
Please wait while we load your content...