Convergent approach to complex spirocyclic pyrans: practical synthesis of the oxa-pinnaic acid core†
Abstract
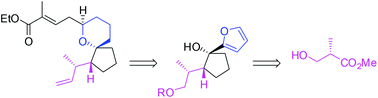
The enantioselective synthesis of the oxa-pinnaic acid framework has been achieved through internal asymmetric induction. The synthetic strategy pursued illustrates the adaptability of the Achmatowicz oxidative rearrangement for the synthesis of complex spirocyclic pyrans starting from tertiary alcohols.

 Please wait while we load your content...
Please wait while we load your content...