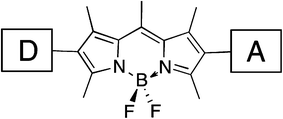
The syntheses and physicochemical properties for a series of 2,6-disubstituted-4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) dyes are reported. The use of chromophores or redox active species as π-spacers, such as BODIPY, requires the inclusion of a sufficiently conjugated donor in order to achieve appropriate charge separation upon photoexcitation. The information derived from this study offers guiding principles for incorporating strongly absorbing, non-innocent π-spacers in organic dye design.
This article is Open Access
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...