Structural modifications of (Z)-3-(2-aminoethyl)-5-(4-ethoxybenzylidene)thiazolidine-2,4-dione that improve selectivity for inhibiting the proliferation of melanoma cells containing active ERK signaling
Abstract
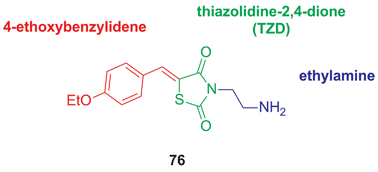
We herein report on the pharmacophore determination of the ERK docking domain inhibitor (Z)-3-(2-aminoethyl)-5-(4-ethoxybenzylidene)thiazolidine-2,4-dione, which has led to the discovery of compounds with greater selectivities for inhibiting the proliferation of melanoma cells containing active ERK signaling.
- This article is part of the themed collection: In Celebration of Andrew D. Hamilton’s Career in Chemistry

 Please wait while we load your content...
Please wait while we load your content...