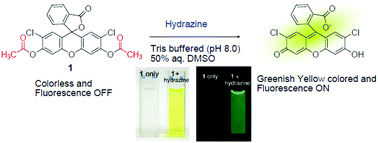
The highly selective chemosignaling behaviors for hydrazine by a reaction-based probe of dichlorofluorescein and resorufin acetates were investigated. Hydrazinolysis of latent dichlorofluorescein and resorufin acetate fluorochromes caused prominent chromogenic and fluorescent turn-on type signals. The probes selectively detected hydrazine in the presence of commonly encountered metal ions and anions as background. Dichlorofluorescein and resorufin acetates selectively detected hydrazine with detection limits of 9.0 × 10−8 M and 8.2 × 10−7 M, respectively. Furthermore, hydrazine was selectively detected over other closely related compounds, such as hydroxylamine, ethylenediamine, and ammonia. As a possible application of the acetate probes, hydrazine signaling in tap water was tested.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...