A sterically demanding organo-superbase avoids decomposition of a naked trifluoromethyl carbanion directly generated from fluoroform†
Abstract
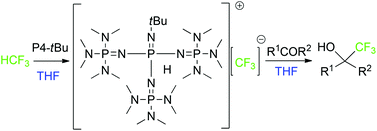
A simple strategy avoiding the decomposition of a naked trifluoromethyl anion to difluorocarbene by a sterically very demanding organo-superbase without the help of a

 Please wait while we load your content...
Please wait while we load your content...