Multi-component synthesis of peptide–sugar conjugates†
Abstract
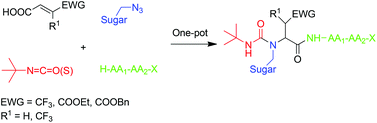
Recent years have witnessed a growing interest in the development of new methods for linking sugars to
* Corresponding authors
a Department of Food, Environmental and Nutritional Sciences, Università degli Studi di Milano, via Celoria 2, 20133 Milano, Italy
b
Department of Chemistry, Materials and Chemical Engineering “Giulio Natta”, Politecnico di Milano, via Mancinelli 7, 20131 Milano, Italy
E-mail:
alessandro.volonterio@polimi.it
Fax: +39 0223993080
Tel: +39 0223993136
Recent years have witnessed a growing interest in the development of new methods for linking sugars to

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
M. C. Bellucci, G. Terraneo and A. Volonterio, Org. Biomol. Chem., 2013, 11, 2421 DOI: 10.1039/C3OB27176E
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
