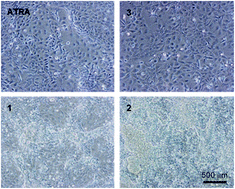
Understanding how the structure of molecules relates to their function and biological activity is essential in the development of new analogues with targeted activity. This is especially relevant in mediating developmental processes in mammalian cells and the regulation of stem cell differentiation. In this study, thiazole-containing small molecules were synthesised and investigated for their ability to induce the differentiation of human pluripotent stem cells and their derivatives. Analyses of cell morphology, cell viability, expression of cell surface markers and ability to induce cell differentiation and regulate neurite formation identified the analogue with the longest and most bulky hydrophobic side chain as possessing comparable or enhanced activity to all-trans-retinoic acid (ATRA). Interestingly, a shorter, less bulky, known thiazole compound reported to be isoform selective for the retinoic acid receptor β2 (RARβ2) agonist did not mediate differentiation under the conditions tested; however, activity could be restored by adjusting the structure to a longer, more bulky molecule. These data provide further insight into the complexity of compound design in terms of developing small molecules with specific biological activities to control the development and differentiation of mammalian cells.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...