Abstract
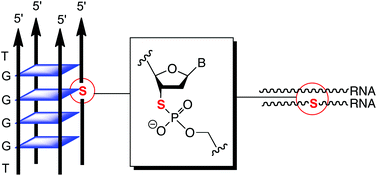
The effect of 3′-S-phosphorothiolate linkages on the stability of RNA·RNA duplexes and G-quadruplex structures has been studied. 3′-Thio-2′-deoxyuridine was incorporated into RNA duplexes and thermal melting studies revealed that the resulting 3′-S-phosphorothiolate linkages increased the stability of the duplex to thermal denaturation. Additionally, and contrary to expectation, a similar effect on duplex stability was observed when the same thionucleoside was incorporated into the RNA strand of a RNA·DNA duplex. A suitably protected derivative of 3′-thio-2′-deoxyguanosine was prepared using an oxidation–reduction strategy and this residue also increased the thermal stability the [d(TGGGGT)]4 G-quadruplex when positioned centrally. The results are discussed in terms of the influence that the sulfur atom has on the conformation of the furanose ring and imply that the previously noted high thermal stability of parallel RNA quadruplexes is not derived from H-bonding interactions of the 2′-hydroxyl group, but can be attributed to conformational effects.
- This article is part of the themed collection: Nucleic acids: new life, new materials

 Please wait while we load your content...
Please wait while we load your content...