Synthesis and structural studies of S-type/N-type-locked/frozen nucleoside analogues and their incorporation in RNA-selective, nuclease resistant 2′–5′ linked oligonucleotides†
Abstract
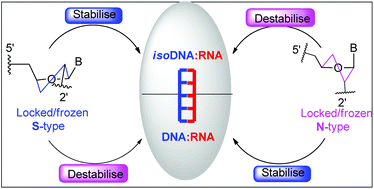
2′-endo locked or frozen (S-type)/3′-endo locked or frozen (N-type)
- This article is part of the themed collection: Nucleic acids: new life, new materials

 Please wait while we load your content...
Please wait while we load your content...