A kinetic study of oxygen reduction reaction and characterization on electrodeposited gold nanoparticles of diameter between 17 nm and 40 nm in 0.5 M sulfuric acid
Abstract
Kinetic and mechanistic studies of the oxygen reduction reaction (ORR) in oxygen saturated 0.5 M
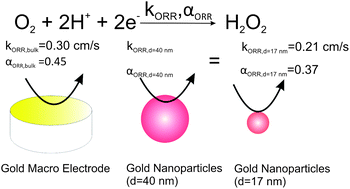

 Please wait while we load your content...
Please wait while we load your content...