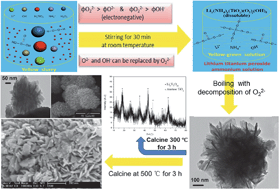
A petal-like nanostructured Li4Ti5O12–TiO2 composite has been synthesized by a novel, simple, ultrafast, low-cost, and environmentally benign process and investigated as an anode material for Li-ion batteries. This work introduces a special solution system not slurry to synthesize a spherical flower-like Li4Ti5O12–TiO2 composite by boiling followed by solid-state calcination at a low temperature of 500 °C for 3 h. Well-crystallized Li4Ti5O12–TiO2 with relatively larger amounts (18.80 weight%) of anatase TiO2 can be obtained, which is different from conventional carbon coating, metal doping and trace TiO2 coating. Owing to the relatively low calcined temperature, the flower-like shape of the precursor powder is maintained after post-treatment. Because of the rough and porous nanostructured particles and mutually complementary intrinsic advantages between Li4Ti5O12 and TiO2, the Li4Ti5O12–TiO2 composite obtained at a relatively low calcined temperature shows excellent electrochemical performance. There are two pairs of long and flat voltage plateaus during charge and discharge at 0.1 C rate, which are not shown in other Li4Ti5O12–TiO2 composites. The initial discharge capacities are 185.5, 177.2, 167.3, 145.8, 137.7, 127.5 and 112.5 mA h g−1 at the 0.1, 0.5, 1, 2, 5, 10 and 20 C rates, respectively. After 100 cycles, the prepared Li4Ti5O12 retains 99.6%, 98.0%, 98.2% and 97.1% of its initial discharge capacities at the 2, 5, 10 and 20 C rates, respectively. After total 450 cycles at 1, 2, 5, 10 and 20 C rates, the cell returns to C/10 and still exhibits a remarkably high discharge capacity of 175.8 mA h g−1 which is 94.8% of the initial discharge capacity at 0.1 C rate.

 Please wait while we load your content...
Please wait while we load your content...