Direct growth of NiCo2O4 nanostructures on conductive substrates with enhanced electrocatalytic activity and stability for methanol oxidation†
Abstract
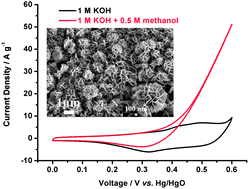
In this report, NiCo2O4 nanostructures with different morphologies were directly grown on conductive substrates (stainless steel and ITO) by a facile electrodeposition method in addition to a post-annealing process. The morphology changes on different conductive substrates are discussed in detail. The NiCo2O4 on stainless steel (SS) had a high surface area (119 m2 g−1) and was successfully used in the electrocatalytic oxidation of methanol. The electrocatalytic performance was investigated by cyclic voltammetry (CV), chronoamperometry and electrochemical impedance spectroscopy (EIS) measurements. Impressively, the NiCo2O4 showed much higher electrocatalytic activity, lower overpotential and greater stability compared to that of only NiO or Co3O4 synthesized by the same method. The higher electrocatalytic activity is due to the high electron conductivity, large surface area of NiCo2O4 and the fast ion/electron transport in the electrode and at the electrolyte–electrode interface. This is important for further development of high performance non-platinum electrocatalysts for application in direct methanol fuel cells.
 Please wait while we load your content...
Please wait while we load your content...