Influence of precursor concentration, surfactant and temperature on the hydrothermal synthesis of CuS: structural, thermal and optical properties
Abstract
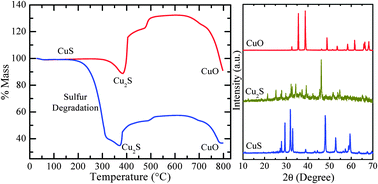
Herein we demonstrate the synthesis and formation mechanism of copper sulfide (CuS and Cu2S) by a simple hydrothermal process in the temperature range of 150–250 °C. The molar concentration ratio of copper nitrate to sodium thiosulfate precursor, and the reaction temperature play important roles in determining the morphology and composition of the product. At a higher thiosulfate concentration, elemental sulfur precipitates out along with CuS. However, the elemental sulfur is degraded with increasing of the reaction temperature. The morphology of the as-synthesized product is primarily a sphere-like agglomeration of either nanoparticles and/or nanoplates. The surfactant is found to be indispensable for obtaining dispersed nanocrystals. The stability of CuS and the formation of different copper compounds at higher temperature are studied in detail using thermal analysis and X-ray diffraction (XRD). The phase pure CuS is found to be completely converted to CuO upon calcining in air at 800 °C. However, the in situ high temperature XRD measurements in a vacuum show complete conversion of CuS to Cu2S at 600 °C without any intermediate phases. The optical bandgap of the as-synthesized CuS nanocrystals is measured to be ∼1.7 eV.

 Please wait while we load your content...
Please wait while we load your content...