Conformational properties of peptides incorporating a fluorinated pseudoproline residue†
Abstract
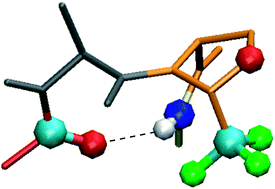
We have recently reported the synthesis of enantiomerically pure CF3-oxazolidine pseudoprolines (CF3-ΨPro). Complete
- This article is part of the themed collection: Prolines

 Please wait while we load your content...
Please wait while we load your content...