Microwave assisted synthesis, spectroscopic characterization and biological aspects of some new chromium(iii) complexes derived from N⁁O donor Schiff bases
Abstract
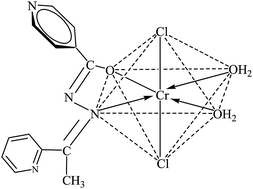
The present study is structured to address the development of green microwave processes in the manufacture of a new series of biologically potent (N⁁O) donor

 Please wait while we load your content...
Please wait while we load your content...