Adsorption of quaternary amine surfactants and their penetration into the intracrystalline cavities of sepiolite
Abstract
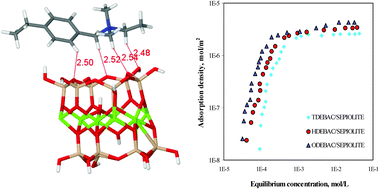
Organoclays are very important composite materials used in many diverse industries including plastics and paint. In this study, an organoclay was formed using sepiolite and homologs of quaternary amine surfactants of tetradecyl dimethyl ethylbenzyl ammonium chloride (TDEBAC), hexadecyl dimethyl ethylbenzyl ammonium chloride (HDEBAC) and octadecyl dimethyl ethylbenzyl ammonium chloride (ODEBAC). Adsorption isotherms of sepiolite/quaternary amine surfactants show that the surfactant with the longest chain, ODEBAC, is adsorbed at most onto the surface of sepiolite. FTIR spectroscopy of organosepiolite indicated that spectroscopic features related to the surface and tunnel of sepiolite are notably modified in the presence of surfactants. In addition to these experiments, high level ab initio counter-poise corrected binding energy computations at MP2, SCS-MP2, B97-D and B2PLYP-D levels of theory employing the TZVP basis set were also performed. At all levels, basal interaction of the surfactant at the surface of sepiolite was found to exhibit the most favourable configuration. Furthermore, penetration of the surfactant into the intra-crystalline cavities of sepiolite was also found to be plausible.

 Please wait while we load your content...
Please wait while we load your content...