Abstract
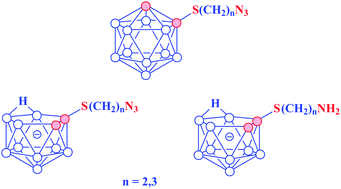
Novel carboranyl amines [7-NH2(CH2)nS-7,8-C2B9H11]− were synthesized by alkylation of 1-mercapto-ortho-carborane with ω-bromoalkylphthalimides followed by removal of the protecting group with hydrazine. closo-Carboranyl azides 1-N3(CH2)nS-1,2-C2B10H11 were prepared by the reaction of the corresponding carboranyl alcohol/iodide with sodium azide and converted to the water soluble nido-form [7-N3(CH2)nS-7,8-C2B9H11]− with ammonium formate in refluxing methanol.

 Please wait while we load your content...
Please wait while we load your content...