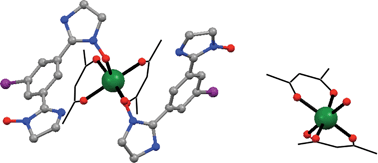
1-Iodo-3,5-bis(4′,4′,5′,5′-tetramethyl-4′,5′-dihydro-1H-imidazole-1′-oxyl)benzene (IPhIN = L) forms co-crystalline [M(hfac)2(IPhIN)2]·[M(hfac)2] coordination complexes (M = Mn, Co) with [Mn(hfac)2] and [Co(hfac)2]. In each system only one of the iminoylnitroxide radical moieties is coordinated as part of the [M(hfac)2(IPhIN)2] structural units; the metal ions do not coordinate the radical nitrogen atoms, but rather the nitroxide oxygen to form a cis NO–M–ON grouping in a hexacoordinate ligand sphere. Solvate water molecules in the lattice provide an important driving force for assembly of the co-crystal structures in the cobalt(II) derivative; an additional factor is the formation of halogen bonds between hfac CF3 groups and the IPhIN iodine atom. The paramagnetic susceptibility (χM) versus temperature (T) measurements showed antiferromagnetic exchange downturns at lower temperature. Based on the crystal structures of the complexes, the χMT versus T data were modelled using a paramagnetic contribution from the [M(hfac)2] fragment, an exchange interaction J1 within the L–M–L fragment, plus an intramolecular radical–radical interaction J2 within the IPhIN biradical unit. For the Mn(II) complex, a good fit was obtained for gMn = 2.14(1), grad = 2.0 (fixed), J1 = −311(9) cm−1 and J2 = 11.1(7) cm−1. A reasonable fit for the cobalt compound was obtained for gCo = 2.72(1), grad = 2.0 (fixed), J1 = −400(5) cm−1, J2 = 28.3(3) cm−1 with a generalized zero field splitting parameter D = 64(2) cm−1.

 Please wait while we load your content...
Please wait while we load your content...