Zinc(ii) complexes with the quinolone antibacterial drug flumequine: structure, DNA- and albumin-binding†
Abstract
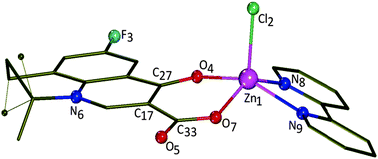
The interaction of Zn(II) with the quinolone antibacterial drug flumequine (Hflmq) in the presence or absence of an N,N′-donor heterocyclic ligand, 2,2′-bipyridine (bipy), is being investigated. Interaction of equimolar quantities of ZnCl2 with flumequine and 2,2′-bipyridine results in the formation of a structurally characterized [Zn(flmq)(bipy)Cl] (2) complex, while excess of flumequine leads to a structurally characterized [Zn(flmq)2(bipy)] (3) compound. The reaction of ZnCl2 with flumequine in the absence of 2,2′-bipyridine leads to formation of complex [Zn(flmq)2(H2O)2] (1). In all these complexes, the deprotonated bidentate flumequinato ligands are coordinated to zinc ions through pyridone and carboxylato oxygens. The complexes exhibit good binding propensity to human or bovine serum albumin protein having relatively high binding constant values. UV study of interaction of the complexes with calf-thymus DNA (CT DNA) has shown that they bind to CT DNA and [Zn(flmq)(bipy)Cl] exhibits the highest binding constant. A competitive study with ethidium bromide (EB) has shown that the complexes can displace DNA-bound EB, indicating that they bind to DNA in strong competition with EB. The complexes bind to CT DNA in an intercalative binding mode which has also been verified by DNA solution viscosity measurements. DNA electrophoretic mobility experiments showed that all complexes bind to pDNA possibly in an intercalative manner resulting in catenanes formation as well as in double-stranded cleavage reflecting (or ending) in the formation of linear DNA.

 Please wait while we load your content...
Please wait while we load your content...