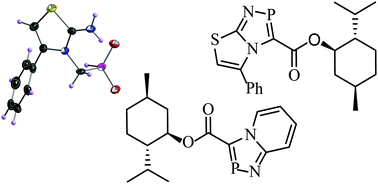
Five new anellated 4H-1,4,2-diazaphospholes including the first examples of chiral 4H-1,4,2-diazaphospholes with the (−)-menthyl substituent have been prepared by two main routes: Hantzsch type cyclocondensation of 4-phenyl-2-amino-1,3-thiazole with chloromethyl dichlorophosphine and 4+1 cyclocondensation of cycloimmonium salts derived from 4-phenyl-2-amino-1,3-thiazole and 2-aminopyridine with PCl3 and NEt3. The new compounds have been characterized by multinuclear (1H, 13C, 31P) NMR spectroscopy. Controlled hydrolysis of 5-phenylthiazolo[3,2-d][1,4,2]diazaphosphole 2a with two equivalents of water yields the corresponding zwitterionic phosphinate 9, the structure of which was elucidated by single crystal X-ray diffraction. The structure in the solid state is governed by strong N–H⋯O hydrogen bonding resulting in chains along the crystallographic a-axis. The chloroform molecules are integrated in the chains by weaker C–H⋯O bonds and C–Cl⋯C halogen bonds.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...