Oxaliplatin complexes with carnosine and its derivatives: in vitro cytotoxicity, mass spectrometric and computational studies with a focus on complex fragmentation†
Abstract
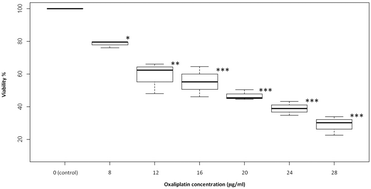
The complexation of the Pt-based anti-cancer drug oxaliplatin (OxPt) with biological ligands other than DNA is believed to be a major cellular sink for the drug reducing its therapeutic potential and acting as a potential cause of toxicity. In this paper, the very first hypothesis driven investigation of the role of the naturally abundant cytoplasmic dipeptide ligand β-alanyl-L-histidine dipeptide (carnosine) in OxPt detoxification is presented. In vitro studies on hepatocellular carcinoma HepG2 cells suggest that carnosine may inhibit the cytotoxic action of OxPt most likely through the formation of complexes that are less cytotoxic than OxPt alone. Evidence is provided to suggest that pre-exposure of HepG2 cells to elevated levels of carnosine appears to have a lasting effect on reducing the cytotoxicity of OxPt even after the removal of the externally added carnosine. This effect, however, is likely under kinetic control as its magnitude was shown not to vary significantly with the level of carnosine exposure within the concentration range used in this study. Various mass spectrometry techniques employing electrospray ionization and chip nanospray were employed to study the interaction of oxaliplatin with carnosine as well as two of its derivatives β-alanyl-N-methylhistidine (anserine) and N-acetylcarnosine (NAC). Evidence of complexation between OxPt and each of the three ligands examined is presented. Most species observed were unambiguously assigned and compared to their theoretical isotopic patterns. Common fragmentation products due to the collisionally-activated protonated complexes of each of the ligands examined with OxPt, [M + OxPt + H]+, where M = carnosine, anserine or NAC, were reported. Density functional calculations at the B3LYP/LANL2DZ level were used to obtain structural information and relative free energies of different isomers of the observed precursor [Carnosine + OxPt + H]+ both in the gas phase and in solution as well as to probe its fragmentation, highlighting plausible fragmentation mechanisms that account for all the experimental results. Data are presented to show several binding modes between electron rich sites such as N and O centers of carnosine and the Pt metal of OxPt. Calculations were also employed to obtain proton affinities and free energies of key reactions. The proton affinities of carnosine, anserine and NAC at 298 K were calculated to be 254.4, 255.9 and 250.2 kcal mol−1 respectively. To the best of our knowledge the proton affinities of anserine and N-acetyl-carnosine are the first reported values in the literature.

 Please wait while we load your content...
Please wait while we load your content...