The role of glutathione in the metabolism of diphenylarsinic acid in rats†
Abstract
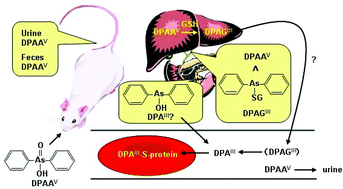
Diphenylarsinic acid (DPAAV) is a chemical precursor as well as a degradation product of arsenic-containing chemical weapons such as Clark 1 (diphenylarsine chloride) and Clark 2 (diphenylarsine cyanide). Compared to inorganic arsenicals, toxicological findings on DPAAV are limited. To elucidate the mechanism of DPAAV toxicity, we investigated the metabolic behavior of DPAAV in the body. Specifically, we examined the distribution and biliary excretion of DPAAV and its metabolites in rats orally administered DPAAV at a dose of 1.0 mg As kg−1 body weight. DPAAV was excreted in bile, either as the original DPAAV or as a DPAA–GSH complex (DPAGIII), as determined by HPLC-ICP-MS and HPLC-ESI-MS, with DPAGIII being the main chemical form. Approximately 1.7% and 2.4% of the dose was accumulated in erythrocytes three hours and three days after administration, respectively. Approximately 91% of the dose was excreted in urine and feces as DPAAV in three days, mostly in the urine. Accumulation of arsenic in erythrocytes was investigated in vitro by incubating (at 37 °C for up to three hours) DPAAV or DPAGIII with a suspension of rat erythrocytes (10% in Tris-HCl-buffered saline). Approximately 80% of the DPAGIII dose was taken up by erythrocytes within thirty seconds, while approximately 35% of the DPAAV dose was taken up by erythrocytes after three hours of incubation. DPAGIII was possibly hydrolyzed and converted to trivalent unconjugated arsenical (diphenylarsinous acid, DPAIII), which also was accumulated in erythrocytes. As expected, DPAIII exhibited greater affinity for erythrocyte proteins than did DPAAV.
- This article is part of the themed collection: Metallomics in Japan, 2013

 Please wait while we load your content...
Please wait while we load your content...