Development and evaluation of selective, reversible LSD1 inhibitors derived from fragments†
Abstract
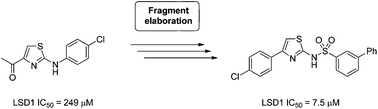
Two series of aminothiazoles have been developed as reversible inhibitors of lysine specific demethylase 1 (LSD1) through the expansion of a hit derived from a high concentration biochemical fragment based screen of 2466 compounds. The potency of the initial fragment hit was increased 32-fold through synthesis, with one series of compounds showing clear structure–activity relationships and inhibitory activities in the range of 7 to 187 μM in a biochemical assay. This series also showed selectivity against the related FAD-dependent enzyme mono-amine oxidase A (MAO-A). Although a wide range of irreversible inhibitors of LSD1 have been reported with activities in the low nanomolar range, this work represents one of the first reported examples of a reversible small molecule inhibitor of LSD1 with clear SAR and selectivity against MAO-A, and could provide a platform for the development of more potent reversible inhibitors. Herein, we also report the use of a recently developed cell-based assay for profiling LSD1 inhibitors, and present results on our own compounds as well as a selection of recently described reversible LSD1 inhibitors.
 Please wait while we load your content...
Please wait while we load your content...