Ring opening polymerization of mannosyl tricyclic orthoesters: rationalising the stereo and regioselectivity of glycosidic bond formation using quantum chemical calculations†
Abstract
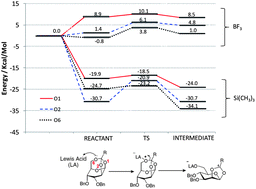
Quantum chemical calculations have been used to assess the physico-chemical origin of the stereo and regio-selectivity of polymerisation reactions of glycosyl tricyclic orthoesters. From the theoretical reaction pathway we find that subtle modulation of steric and electronic effects at the initiation event can dramatically influence the nature of the

 Please wait while we load your content...
Please wait while we load your content...