Design and biological evaluation of synthetic retinoids: probing length vs. stability vs. activity
Abstract
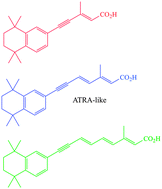
All trans-retinoic acid (ATRA) is widely used to direct the differentiation of cultured stem cells. When exposed to the pluripotent human embryonal carcinoma (EC) stem cell line, TERA2.cl.SP12, ATRA induces ectoderm differentiation and the formation of neuronal cell types. We report in this study that novel polyene chain length analogues of ATRA require a specific chain length to elicit a biological responses of the EC cells TERA2.cl.SP12, with synthetic retinoid AH61 being particularly active, and indeed more so than ATRA. The impacts of both the synthetic retinoid AH61 and natural ATRA on the TERA2.cl.SP12 cells were directly compared using both RT-PCR and Fourier Transform Infrared Micro-Spectroscopy (FT-IRMS) coupled with multivariate analysis. Analytical results produced from this study also confirmed that the synthetic retinoid AH61 had biological activity comparable or greater than that of ATRA. In addition to this, AH61 has the added advantage of greater compound stability than ATRA, therefore, avoiding issues of oxidation or decomposition during use with embryonic stem cells.

 Please wait while we load your content...
Please wait while we load your content...