Characterization of a nanogland for the autotransplantation of human pancreatic islets†
Abstract
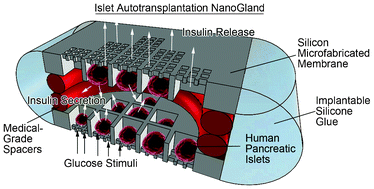
Despite the clinical success of pancreatic islet transplantation, graft function is frequently lost over time due to islet dispersion, lack of neovascularization, and loss of physiological architecture. To address these problems, islet encapsulation strategies including scaffolds and devices have been developed, which produced encouraging results in preclinical models. However, islet loss from such architectures could represent a significant limitation to clinical use. Here, we developed and characterized a novel islet encapsulation silicon device, the NanoGland, to overcome islet loss, while providing a physiological-like environment for long-term islet viability and revascularization. NanoGlands, microfabricated with a channel size ranging from 3.6 nm to 60 μm, were mathematically modeled to predict the kinetics of the response of encapsulated islets to

 Please wait while we load your content...
Please wait while we load your content...