Product qualification: a barrier to point-of-care microfluidic-based diagnostics?
Abstract
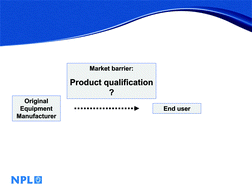
One of the most exciting applications of microfluidics-based diagnostics is its potential use in next generation point-of-care (POC) devices. Many prototypes are already in existence, but, as of yet, few have achieved commercialisation. In this article, we consider the issue surrounding product qualification as a potential barrier to market success. The study discusses, in the context of POC microfluidics-based diagnostics, what the generic issues are and potential solutions. Our findings underline the need for a community-based effort that is necessary to speed up the product qualification process.

 Please wait while we load your content...
Please wait while we load your content...