Chip-based liver equivalents for toxicity testing – organotypicalness versus cost-efficient high throughput
Abstract
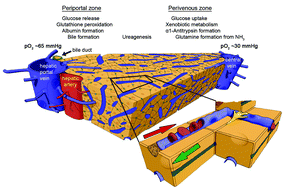
Drug-induced liver toxicity dominates the reasons for pharmaceutical product ban, withdrawal or non-approval since the thalidomide disaster in the late-1950s. Hopes to finally solve the liver toxicity test dilemma have recently risen to a historic level based on the latest progress in human microfluidic tissue culture devices. Chip-based human liver equivalents are envisaged to identify liver toxic agents regularly undiscovered by current test procedures at industrial throughput. In this review, we focus on advanced microfluidic microscale liver equivalents, appraising them against the level of architectural and, consequently, functional identity with their human counterpart in vivo. We emphasise the inherent relationship between human liver architecture and its drug-induced injury. Furthermore, we plot the current socio-economic drug development environment against the possible value such systems may add. Finally, we try to sketch a forecast for translational innovations in the field.
- This article is part of the themed collection: Organs on a Chip 2013

 Please wait while we load your content...
Please wait while we load your content...