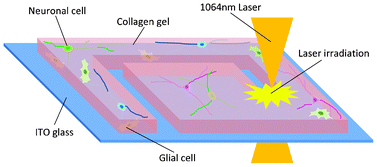
Two-dimensional (2D) micropatterning techniques have been developed to guide dissociated neurons into predefined distributions on solid substrates, such as glass and plastic. Micropatterning methods using three-dimensional (3D) substrates or scaffolds that reproduce aspects of the in vivo microenvironment could facilitate the engineering of functional tissues for transplantation or more robust experimental models. We developed a 3D collagen gel photothermal etching method using an infrared laser that precisely controls the area of cell adhesion and neurite projection by etching a small targeted section of the collage gel. It was then possible to guide neural network formation under microscopic observation. After conventional cell seeding, we succeeded in creating isolated 3D networks, while controlling (1) the number of each neural subtype (neurons, glia, and fluorescently-labeled neurons) and (2) the direction of neurite elongation. Neurons seeded on a 10-μm-thick collagen gel survived longer and projected greater numbers of neurites than neurons growing on 2D culture substrates. Intracellular Ca2+ imaging revealed both synchronous and discordant oscillations in different neuronal populations that suggested the pattern and strength of synaptic connectivity. This photothermal etching technique allows for the creation of designed 3D neural networks during cultivation for use in studies of synaptic transmission, neuron–glial signaling, pathogenesis, and drug responses.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...