Reclamation and reuse of ionic liquids from silica-based ionogels using spontaneous water-driven separation†
Abstract
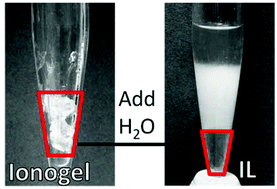
Ionic liquids (ILs) are widely regarded as “green” materials, partially because they are assumed to be recyclable in most applications. Here, the effectiveness of a water-based IL reclamation process with low energy requirements is demonstrated using two types of silica-supported ionic liquid-based gel electrolytes (ionogels) incorporating a variety of common ionic liquids. More than 90% of the IL can be separated from the ionogel silica scaffold simply by submerging the ionogel in water, with no additional energy input. It is posited that water is able to compete with the hydrophobic IL for access to the hydrophilic silica scaffold surface, liberating the IL from the ionogel structure. Spontaneous water-based separation leads to the recovery of at least 70% of the original IL mass; transfer losses experienced at the laboratory scale are expected to decrease with process scale-up. Recovered ILs exhibit similar electrical performance to the virgin materials in a double layer capacitor structure.

 Please wait while we load your content...
Please wait while we load your content...