Engaging isatins in solvent-free, sterically congested Passerini reaction†
Abstract
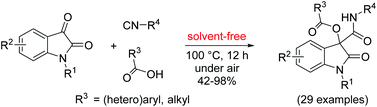
A facile, atom-economic and environmentally-benign protocol for the synthesis of biologically important oxindole derivatives in high yields has been demonstrated by employing isatins as carbonyl compound surrogates in a Passerini reaction carried out under solvent-free conditions. Moreover, electron-deficient phenols can also be used as the acid component in this reaction. In addition, the synthetic utility of the present methodology was examined by the one-pot synthesis of oxindoles with a free –OH group at the benzylic position.

 Please wait while we load your content...
Please wait while we load your content...