Modulation of amyloid-β 1-42 structure and toxicity by proline-rich whey peptides
Abstract
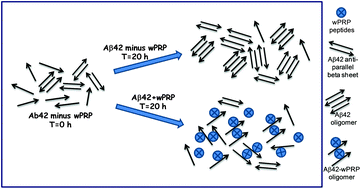
A proline-rich
* Corresponding authors
a CSIRO Preventative Health Flagship, Material Science and Engineering, 343 Royal Parade, Parkville, Victoria, Australia
b CSIRO Preventative Health National Research Flagship, PO Box 10041, Adelaide, BC, SA, Australia
c Centre of Excellence for Alzheimer's Disease Research & Care, School of Exercise, Biomedical & Health Sciences, Edith Cowan University, Australia
d Centre de Biologie Structurale et de Bioinformatique Faculte des Sciences, Universite Libre de Bruxelles Boulevard du Triomphe, Bat. BC, Niveau 4, Zip: 1050 Brussels, Belgium
e
CSIRO Preventative Health Flagship, Food and Nutritional Sciences, 671 Sneydes Road, Werribee, Victoria, Australia
E-mail:
louise.bennett@csiro.au
A proline-rich

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
P. Bharadwaj, R. Head, R. Martins, V. Raussens, R. Sarroukh, H. Jegasothy, L. Waddington and L. Bennett, Food Funct., 2013, 4, 92 DOI: 10.1039/C2FO30111C
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
