Fingerprinting the oxidation state of U(iv) by emission spectroscopy†
Abstract
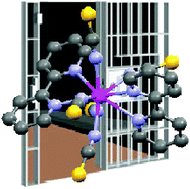
The solid-state structure of the known complex [Et4N][U(NCS)5(bipy)2] has been re-determined and a detailed spectroscopic and magnetic study has been performed in order to confirm the oxidation states of both metal and bipy ligand. Electronic absorption and infrared spectroscopy suggest that the uranium is in its +4 oxidation state and this has been corroborated by emission spectroscopy and variable temperature magnetic measurements, as well as theoretical calculations. Therefore the bipy ligands are neutral, innocent ligands and not, as would be inferred from just a solid state structure, radical anions.

 Please wait while we load your content...
Please wait while we load your content...