Synthesis and characterization of ruthenium polypyridyl complexes with hydroxypyridine derivatives: effect of protonation and ethylation at the pyridyl nitrogen†
Abstract
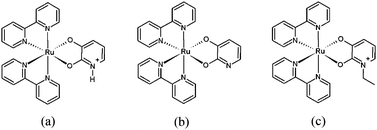
A new series of ruthenium polypyridyl complexes with a hydroxypyridine ligand were prepared, and their properties were investigated spectroscopically and electrochemically. Particular focus is paid to the effects of protonation–deprotonation and ethylation of the hydroxypyridine ligand, which affects the NMR, electronic spectroscopy, and electrochemistry of the complex. The changes to the UV-vis spectrum were used to determine a pka of 10.5 for the hydroxypyridine nitrogen. In the NMR, protonation of the hydroxypyridine ligand of the complex causes changes in the chemical shifts of the protons on both the hydroxypyridine and bipyridine rings, indicating some degree of electronic communication between these ligands. In addition, it is found that deprotonation of the hydroxypyridine ligand strongly affects the redox potential of the ruthenium metal center, shifting it more negative by 0.4 V. While the electrochemistry of the protonated complex contains irreversible electrochemical events, both deprotonation and subsequent ethylation of the hydroxypyridine ligand result in reversible electrochemistry for all events within the solvent window. For the ethylated complex, we search for a ligand to ligand charge transfer band, corresponding to electron transfer between bipyridine ligands in the mixed valence state. Despite the potential for electronic coupling between ligands through the metal center, we were unable to find any spectroscopic evidence of such electronic coupling.

 Please wait while we load your content...
Please wait while we load your content...