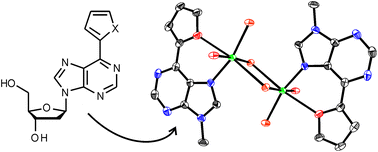
The artificial nucleobases 6-(2′-thienyl)-purine (6TP) and 6-(2′-furyl)-purine (6FP) have been investigated in terms of their applicability in metal-mediated base pairs. In principle, these nucleobases should be capable of providing an S,N- and O,N-coordination environment, respectively. Two metal complexes have been synthesized and structurally characterized, using the N9-methylated derivatives of 6TP and 6FP as model nucleobases. The silver(I) complex [Ag(9-Me6TP)(CH3CN)(NO3)]n is of polymeric nature. The thienyl substituent is not involved in metal binding. In contrast, the dinuclear copper(II) complex [Cu(9-Me6FP)Cl2(OH2)]2 clearly shows that the furyl substituent can participate in the coordination of the metal ion. Oligonucleotide double helices comprising a 6TP:6TP or a 6FP:6FP mispair are slightly stabilized in the presence of silver(I). However, a distinction between the formation of a metal-mediated base pair and an unspecific binding event cannot be finally made. Based on these studies, it is proposed that the formation of Hoogsteen-type metal-mediated base pairs should be the more promising alternative compared with the originally anticipated Watson–Crick-type metal-mediated base pairs.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...