Novel porphyrin–MCp*(dppe) conjugates with the acetylene linker, por-C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CMCp*(dppe) (por = (5,15-diarylporphinato)zinc(II), Cp* = η5-C5Me5, dppe = 1,2-bis(diphenylphosphino)ethane; M/aryl = Fe/phenyl (1), Fe/3,5-di-tert-butylphenyl (2), Ru/phenyl (3)), are synthesized. Absorption and fluorescence spectroscopic studies combined with electrochemical investigations reveal strong interactions between the porphyrin moieties and the electron-donating MCp*(dppe) fragments. Oxidation of the porphyrin core of the iron conjugate 1 generates the dication radical 12+, which is spontaneously associated with its mono-cationic counterpart 1+ to form the stable π-radical trication dimer [12]3+, whereas complexes 2 and 3 undergo simple oxidation without forming such dimers. Furthermore, intramolecular charge transfer between the porphyrin rings and the MCp*(dppe) fragments causes the appearance of a charge transfer absorption band in the visible region. It is also found that fluorescence derived from the porphyrin rings is quenched upon oxidation via intramolecular photoinduced electron transfer from the MCp*(dppe) moieties to the porphyrin chromophores in the excited states. The emission is recovered by subsequent reduction of the MCp*(dppe) fragments. Thus, the fluorescence from the porphyrin moieties is switched off and on upon oxidation and subsequent reduction, respectively. The iron acetylide complex 1 can assemble with nitrogen-donors including pyridine and DABCO as well as a π-acceptor, naphthalenediimide, to provide the nano-sized stacking structures which are detected by NMR.
CMCp*(dppe) (por = (5,15-diarylporphinato)zinc(II), Cp* = η5-C5Me5, dppe = 1,2-bis(diphenylphosphino)ethane; M/aryl = Fe/phenyl (1), Fe/3,5-di-tert-butylphenyl (2), Ru/phenyl (3)), are synthesized. Absorption and fluorescence spectroscopic studies combined with electrochemical investigations reveal strong interactions between the porphyrin moieties and the electron-donating MCp*(dppe) fragments. Oxidation of the porphyrin core of the iron conjugate 1 generates the dication radical 12+, which is spontaneously associated with its mono-cationic counterpart 1+ to form the stable π-radical trication dimer [12]3+, whereas complexes 2 and 3 undergo simple oxidation without forming such dimers. Furthermore, intramolecular charge transfer between the porphyrin rings and the MCp*(dppe) fragments causes the appearance of a charge transfer absorption band in the visible region. It is also found that fluorescence derived from the porphyrin rings is quenched upon oxidation via intramolecular photoinduced electron transfer from the MCp*(dppe) moieties to the porphyrin chromophores in the excited states. The emission is recovered by subsequent reduction of the MCp*(dppe) fragments. Thus, the fluorescence from the porphyrin moieties is switched off and on upon oxidation and subsequent reduction, respectively. The iron acetylide complex 1 can assemble with nitrogen-donors including pyridine and DABCO as well as a π-acceptor, naphthalenediimide, to provide the nano-sized stacking structures which are detected by NMR.
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CMCp*(dppe) (por = (5,15-diarylporphinato)zinc(II), Cp* = η5-C5Me5,
CMCp*(dppe) (por = (5,15-diarylporphinato)zinc(II), Cp* = η5-C5Me5, 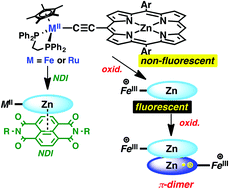

 Please wait while we load your content...
Please wait while we load your content...