Boron azides in Staudinger oxidations and cycloadditions†
Abstract
Staudinger reactions of Cy2BN3 with tri-substituted ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PR3 (R = Et, tBu, Cy, Ph) (1a–1d respectively). Similarly, reaction of (C6F5)2BN3 with the
PR3 (R = Et, tBu, Cy, Ph) (1a–1d respectively). Similarly, reaction of (C6F5)2BN3 with the ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh and Ph2PC
CPh and Ph2PC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPPh2 yielded (C6F5)2BN
CPPh2 yielded (C6F5)2BN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PR3 (2a–d respectively). In contrast, the reaction of (C6F5)2BN3 with Ph2P–C
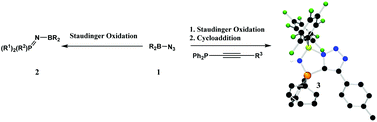
PR3 (2a–d respectively). In contrast, the reaction of (C6F5)2BN3 with Ph2P–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Cp-tol in the presence of excess Me3SiN3 afforded the bicyclic product 3 [1-(C6F5)2B-4-(p-tol)-1H-1,2,3-triazole-5-P(NH)Ph2] in which both a Staudinger and a cycloaddition reaction has taken place. However, if the Staudinger reaction is prohibited via
Cp-tol in the presence of excess Me3SiN3 afforded the bicyclic product 3 [1-(C6F5)2B-4-(p-tol)-1H-1,2,3-triazole-5-P(NH)Ph2] in which both a Staudinger and a cycloaddition reaction has taken place. However, if the Staudinger reaction is prohibited via ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CP(O)(OEt)2 then the unusual dimeric product 4 [2-(C6F5)2B-4-(P(O)OEt2)-2H-1,2,3-triazole-5-P(O)(OEt)(OB(C6F5)2)] is generated. The structures of 1b–d, 2b–d, 3 and 4 have been determined by
CP(O)(OEt)2 then the unusual dimeric product 4 [2-(C6F5)2B-4-(P(O)OEt2)-2H-1,2,3-triazole-5-P(O)(OEt)(OB(C6F5)2)] is generated. The structures of 1b–d, 2b–d, 3 and 4 have been determined by

 Please wait while we load your content...
Please wait while we load your content...