Functionalized arene–ruthenium(ii) complexes: dangling vs. tethering side chain†
Abstract
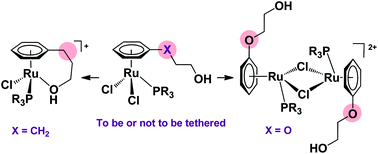
The reactivity of compounds [RuCl2(η6-C6H5OCH2CH2OH)(L)] (L = phosphine or phosphite) towards the chloride abstractor AgSbF6 has been investigated. Thus, the treatment of the triphenylphosphite complex [RuCl2(η6-C6H5OCH2CH2OH){P(OPh)3}] with one equivalent of AgSbF6 gave rise to the formation of the dinuclear dichloro-bridged species [{Ru(μ-Cl)(η6-C6H5OCH2CH2OH){P(OPh)3}}2]2+ as the hexafluoroantimonate salt. On the other hand, the triphenylphosphine analog [RuCl2(η6-C6H5OCH2CH2OH)(PPh3)] led, under the same experimental conditions, to the di-ruthenium derivative [{RuCl(η6-C6H5OCH2CH2OH)(PPh3)}2(μ-Cl)][SbF6] containing only one Cl-bridge. In sharp contrast, treatment of precursors [RuCl2(η6-C6H5CH2CH2CH2OH)(L)] (L = P(OPh)3, PPh3, P(OEt)3) with AgSbF6 resulted in the clean formation of the tethered compounds [RuCl{η6:κ1(O)-C6H5CH2CH2CH2OH}(L)][SbF6]. The differences in reactivity observed have been rationalized by theoretical calculations.

 Please wait while we load your content...
Please wait while we load your content...