Solvent and metal dependent 1H NMR hyperfine shifts in paramagnetic pentaamminemetal cyanide-bridged mixed-valence complexes†
Abstract
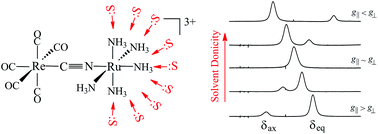
1 H NMR resonances, in several aprotic solvents, are reported for axial and equatorial ammonias coordinated to a single spin paramagnetic centre in the Robin-Day Class II cyanide-bridged mixed-valence cations [(OC)5Cr(μ-CN)M(NH3)5]2+ (M = Ru, Os) as well as in the complex [(OC)5Re(μ-CN)Ru(NH3)5]3+, whose synthesis and properties are reported herein. Using the appropriate isotropic hexaammine complex as a reference, the chemical shift difference between the ammonia protons, δax − δeq, is found to be very sensitive to the paramagnetic metal (M), the remote diamagnetic metal (Cr or Re) and also to the donor properties of the solvent (as well as the counter-ion) as a result of hydrogen bonding interactions. The difference varies linearly with the MMCT energy, and in [(OC)5Re(μ-CN)Ru(NH3)5]3+ can be tuned from positive (δax > δeq) to negative (δax < δeq) through zero (δax = δeq) by the choice of solvent. This reflects the sign and magnitude of the axial ligand field parameter which is in turn a result of changes in the π-donor–acceptor interactions between the donor-cyanide bridging group and the pentaammine metal unit.

 Please wait while we load your content...
Please wait while we load your content...